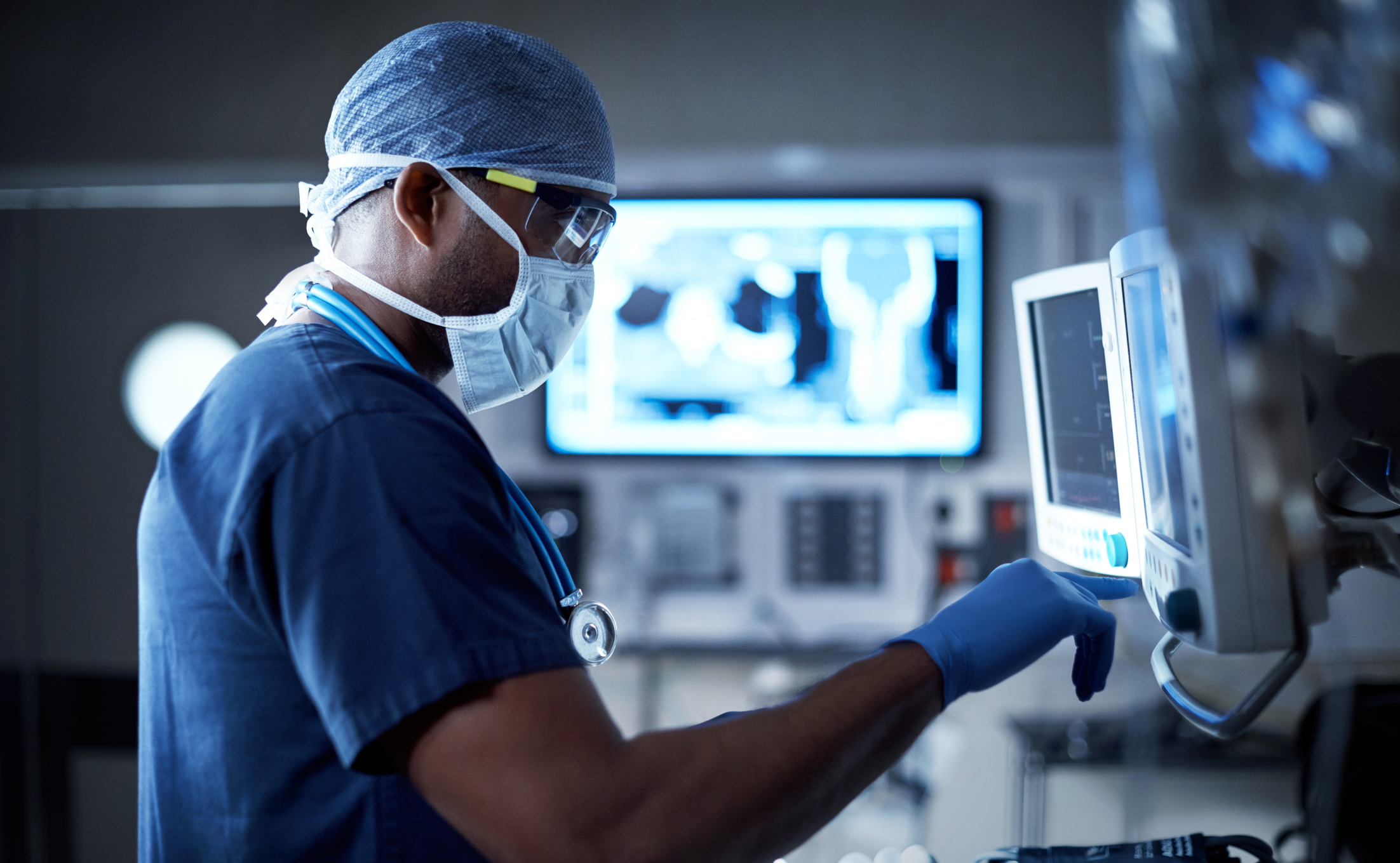
Aversan understands that when it comes to healthcare, safety is of the utmost importance. This is why we aim to boost confidence and support the development of dependable, safe, and industry-leading medical devices. Our proven product development process allows us to mitigate risks and deliver products on-time, on-quality, and on-budget.
Radiation hardening: Electronics that are intended for use in space must be able to withstand the radiation environment of space, which can cause damage to electronic components. Radiation hardening involves designing and manufacturing electronic components and systems that can resist radiation damage, such as using radiation-hardened materials and circuit designs.
Temperature control: Temperature control is critical in space electronics manufacturing, as components can experience extreme temperature swings in space. This requires designing and manufacturing electronics that can operate at both high and low temperatures and implementing thermal management techniques such as heat sinks, thermal coatings, and temperature sensors.
Low power consumption: In space applications, power is at a premium, so space electronics must be designed to minimize power consumption. This involves using low-power components and optimizing system designs to reduce power consumption.
Testing and validation: As with aerospace electronics, space electronics must undergo extensive testing and validation to ensure they meet the necessary performance and safety requirements. This includes testing for radiation hardness, thermal performance, and other key parameters.
Standards compliance: Space electronics must comply with strict standards and regulations set by organizations such as NASA and the European Space Agency. These standards cover a range of topics, including radiation hardness, materials selection, and testing requirements.
Overall, space electronics manufacturing is a highly specialized field that requires deep technical expertise and a commitment to safety and reliability. As space exploration and commercialization continue to expand, the demand for advanced space electronics will only continue to grow, making this an exciting and important field for those interested in electronics and space exploration.

A single source from design concept through product delivery management

Nimble, customizable teams quickly ramped up to support specialized needs

Experienced management, software, electrical, FPGA, and mechanical engineering professionals

Constant evaluation of technical, regulatory, and competitive factors with a focus on reliability, Availability

Shorter development cycles through early identification of gaps and automated testing coverage

Navigating regulatory frameworks and understanding device specific requirements

Aversan was chosen by Laborie Medical Technologies to automate the verification & validation efforts pertaining to Laborie’s medical devices.